The growth has been in secondary batteries (rechargeable) but non-rechargeable or primary batteries are equally important. They continue to fill an important niche market in applications such as wristwatches, remote controls, electric keys and children’s toys. Primary batteries also assist when charging is impractical or impossible, such as military combat, rescue missions and forest-fire services. Other applications of primary batteries are tire pressure gauges in cars and trucks, transmitters for bird tracking, pacemakers for heart patients, intelligent drill bits for mining,as well as light beacons and remote repeater stations. High specific energy, long storage times and operational readiness make this battery well suited for such applications. The battery can be carried to remote locations and used instantly, even after long storage. Most primary batteries are inexpensive, readily available and environmentally friendly.
Carbon-zinc, also known as the Leclanché battery, is the least expensive battery and comes with consumer devices when batteries are included. These general purpose batteries are used for applications with low power drain, such as remote controls, flashlights, children’s toys and wall clocks. One of the most common primary batteries for consumers is the alkaline-manganese, or alkaline for short. Lewis Urry invented it in 1949 while working with the Eveready Battery Company Laboratory in Parma, Ohio. Alkaline delivers more energy at higher load currents than carbon-zinc. Best of all, alkaline does not leak when depleted, as carbon-zinc does. On the negative side, alkaline is more expensive than carbon-zinc.
Primary batteries have one of the highest energy densities. Although secondary batteries have improved, a regular household alkaline provides 50 percent more energy than lithium-ion. The most energy-dense primary is the lithium battery made for film cameras and military combat. It holds more than three times the energy of lithium-ion and comes in various blends, such as lithium-metal, lithium manganese dioxide, lithium-sulfur dioxide, lithium-thionyl chloride, lithium oxygen and others. Figure 1 compares the typical gravimetric energy densities of lead acid, NiMH, Li-ion, alkaline and lithium primary batteries.
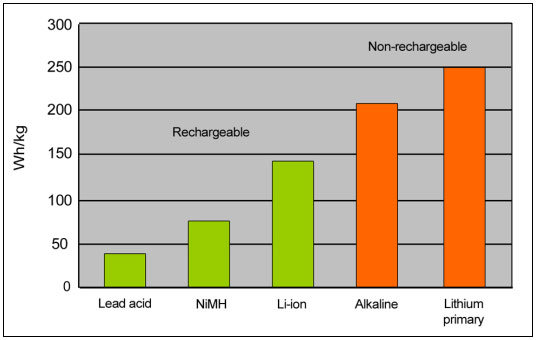
Figure 1: Specific energy comparison of secondary and primary batteries
Secondary batteries are typically rated at 1C; alkaline uses much lower discharge currents.
Secondary batteries are typically rated at 1C; alkaline uses much lower discharge currents.
Courtesy of Cadex
Specific energy indicates the energy a battery can hold. This, however, does not guarantee delivery. Primary batteries tend to have high internal resistance, which limits the discharge to light loads such as remote controls, flashlights and portable entertainment devices. Digital cameras are borderline — a power drill on alkaline would be unthinkable.
Manufacturers of primary batteries only specify specific energy; the specific power (ability to deliver power) is not published. While most secondary batteries are rated at a discharge current of 1C, the capacity of primary batteries is measured by discharging them at a very low current of 25mA, or a fraction of a C. In addition, the batteries are allowed to go down to a very low voltage of 0.8 volts per cell. This evaluation method provides impressive readings on paper, but the results are poor under a more demanding load.
Figure 2 compares performance of primary and secondary batteries on a discharge of 1C. The results are indicated in Actual and Rated. Actual is the Wh/kg derived at a 1C discharge, Rated is the Wh/kg the manufacturer specifies when discharged at a much low current. While the primary batteries do well on a discharge representing entertainment device, secondary batteries have lower capacities but are more resilient at a load of 1C.

Figure 2: Energy comparison under load. ”Rated” refers to a mild discharge; “Actual” is a load at 1C. High internal resistance limits alkaline battery to light loads.
Courtesy of Cadex
The reason for the sharp performance drop on primary batteries is the high internal resistance, which causes the voltage to drop under load. The already high resistance increases further as the battery depletes on discharge. When the battery goes flat on a digital camera, for example, precious capacity is often left behind. A spent alkaline can often power a kitchen clock for two years. Figure 2 above shows the largest discrepancy between “Rated” and “Actual” on alkaline. A long-life alkaline (not shown on chart) will deliver better results.
Table 3 illustrates the capacity of standard alkaline batteries with loads that are typical of personal entertainment devices or small flashlights. Discharging at fractional C-rates produces high capacities; increasing the discharge rate would drastically reduce it.
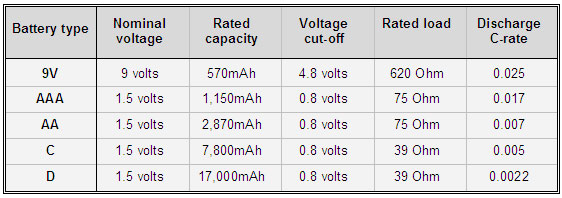
Table 3: Alkaline specifications. The discharge resembles entertainment devices with low loads.
Courtesy of Panasonic
The use of primary batteries can be expensive, and the inability to recharge increases the cost of power by about thirty fold over secondary batteries. The pricing issue becomes even more acute if the packs are being replaced after each mission, regardless of length of service. Discarding partially used batteries is common, especially in fleet applications and critical missions. It is more convenient and safer to simply issue the troops fresh packs with each call rather than estimating the remaining state-of-charge. A US Army general once said that half of the batteries discarded still have 50 percent energy left.
Estimating the battery state-of-charge would help, but such instruments are expensive and inaccurate. The most basic method is measuring the open circuit voltage and reading the internal resistance by applying a brief load and checking the voltage drop. A large voltage differential would relate to rising resistance, a hint to the end of life. A more accurate way is to count the out-flowing energy, a measurement that is also known as coulomb counting, but this requires expensive circuitry. See How to Measure State-of-charge. Due to high cost and inherent inaccuracies, fuel gauges are seldom used on primary batteries.
No comments:
Post a Comment